
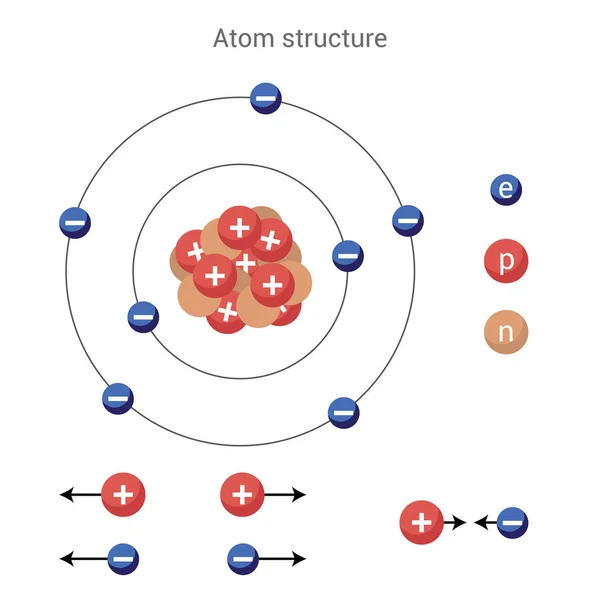
Electrons revolve around the nucleus in circular orbits.Entire mass of the atom is concentrated inside the nucleus.Atom possesses a highly dense, positively charged centre, called nucleus of the order 10 -13cm.It is based upon a-particle scattering experiment. This model could not explain the results of Rutherford scattering experiment. (d) Anti-proton It is negative proton produced by Segre and Weigand (1955).Ītom is a positive sphere with a number of electrons distributed within the sphere. They are unstable particles and include pi ions. (c) Meson Discovered by Yukawa (1935) and Kemmer. (b) Neutrino and antineutrino Particles of small mass and no charge as stated by Fermi (1934). (a) Positron Positive electron ( 0 +1e), discovered by Dirac (1930) and Anderson (1932). Specific charge (e / m) for these rays depends upon the nature of the gas taken and is maximum for H 2.These also cause mechanical motion and are deflected by electric and magnetic field.These travel in straight line and posses mass many times the mass of an electron.Some of the characteristics of anode rays are : (e / m ratio is maximum for hydrogen gas.) The e / m ratio of proton is 9.58 * 10 -4 C / g. It carries a unit positive charge (+1.6 * 10 -19) C). Rutherford discovered proton on the basis of anode ray experiment. These produce X-rays when strike with metal and are deflected by electric and magnetic field.These cause mechanical motion in a small pin wheel placed – their path.These travel in straight line away from cathode and produce fluorescence when strike the glass wall of discharge tube.Some of the characteristics of cathode rays are: Mass of electron is 9.11 * 10 -31 kg and mass of one mole of electron is 0.55 mg. It carries a unit negative charge (-1.6 * 10 -19 C). It contain three subatomic particles namely electrons, protons and neutrons,Įlectron was discovered as a result of study of cathode rays by JJ Thomson. Atomic radii are of the order of 10 -8cm. John Dalton proposed (in 1808) that atom is the smallest indivisible particle of matter.
So, go ahead and check the Important Notes for CBSE Class 11 Chemistry Atomic Structure from this article. With the help of Notes, candidates can plan their Strategy for particular weaker section of the subject and study hard. Candidates who are pursuing in CBSE Class 11 CHemistry are advised to revise the notes from this post.


 0 kommentar(er)
0 kommentar(er)
